
Global Hepatitis E Diagnostic Tests Market Size, Share, Trends, Industry Analysis Report
: Information By Test Type (ELISA HEV IgM Test, ELISA HEV IgG Test, Rapid Diagnostics Test, and RT-PCR), By End User (Hospitals, Diagnostic Laboratories, Research Centers, and Point-of-Care), and By Region (North America, Europe, Asia-Pacific, Latin America, and Middle East & Africa) – Market Forecast, 2024 - 2032
- Published Date:Jul-2024
- Pages: 118
- Format: PDF
- Report ID: PM4998
- Base Year: 2023
- Historical Data: 2019-2022
Report Outlook
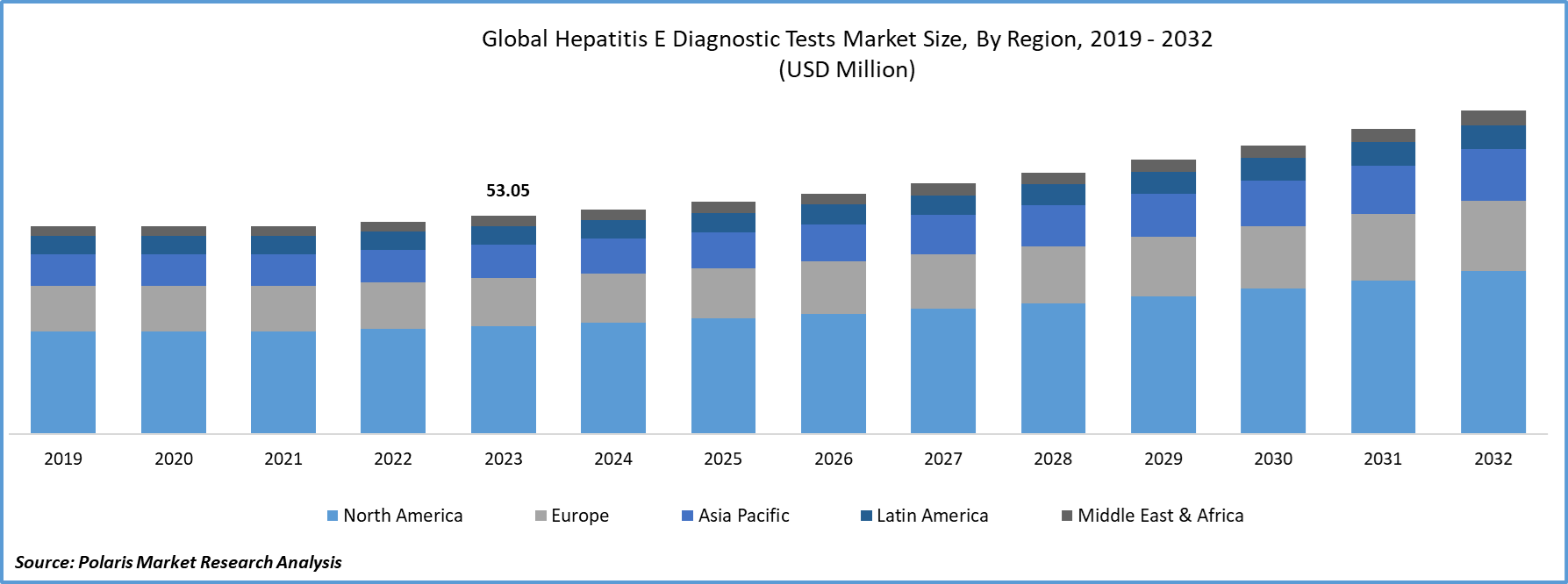
Global Hepatitis E Diagnostic Tests Market Size was valued at USD 53.05 million in 2023. The Hepatitis E Diagnostic Tests industry is projected to grow from USD 54.61 million in 2024 to USD 78.62 million by 2032, exhibiting a compound annual growth rate (CAGR) of 4.6% during the forecast period (2024 - 2032).
Global Hepatitis E diagnostic tests market is growing due to the rising incidence of Hepatitis E, particularly in developing regions where sanitation is poor and water contamination is common. Advancements in diagnostic technologies, including PCR-based assays and point-of-care testing kits, are enhancing early detection and management of the disease. Two key drivers of this market are the increasing prevalence of Hepatitis E, which necessitates more robust diagnostic solutions, and the growing awareness and governmental efforts in disease prevention and control. Notable trends include the integration of advanced molecular diagnostics and the expansion of diagnostic services in emerging markets to improve accessibility and affordability.
To Understand More About this Research: Request a Free Sample Report
The increasing investment in healthcare infrastructure and the focus on infectious disease management are also contributing to the growth of the Hepatitis E diagnostic tests market. Collaborations between governmental bodies, non-profit organizations, and private companies are fostering the development and distribution of more efficient and cost-effective diagnostic tools. Additionally, technological innovations, such as the use of AI and machine learning in diagnostic processes, are expected to streamline Hepatitis E detection further and improve patient outcomes. These efforts are crucial in addressing the global burden of Hepatitis E and mitigating its impact on public health.
Hepatitis E Diagnostic Tests Market Trends
Advancements in Diagnostic Technologies
One major trend in the global Hepatitis E diagnostic tests market is the rapid advancement in diagnostic technologies. Traditional diagnostic methods are being complemented and increasingly replaced by advanced molecular diagnostics, including PCR-based assays and next-generation sequencing (NGS). These technologies offer higher sensitivity and specificity, allowing for earlier and more accurate detection of Hepatitis E virus (HEV) infections. Point-of-care testing (POCT) kits, which provide rapid results, are also gaining traction, especially in resource-limited settings. The integration of these advanced technologies is not only improving diagnostic accuracy but also enabling more effective disease management and control.
Expansion in Emerging Markets
Another significant trend is the expansion of diagnostic services in emerging markets. Countries in Asia, Africa, and Latin America, where Hepatitis E is more prevalent, are witnessing increased efforts to improve healthcare infrastructure and diagnostic capabilities. Governments and international health organizations are investing in expanding access to diagnostic tools and services to manage better and control the spread of HEV. This trend is driven by the growing recognition of the public health impact of Hepatitis E and the need for timely and accurate diagnostics to reduce the disease burden. Improved accessibility to diagnostic services in these regions is expected to play a crucial role in mitigating the impact of Hepatitis E.
Integration of AI and Machine Learning
The integration of artificial intelligence (AI) and machine learning (ML) in the diagnostic process is a transformative trend in the Hepatitis E diagnostic tests market. AI and ML algorithms are being developed to analyze diagnostic data more efficiently, providing insights that enhance the accuracy and speed of HEV detection. These technologies can identify patterns and anomalies that might be missed by traditional diagnostic methods, leading to earlier diagnosis and improved patient outcomes. Moreover, AI-driven diagnostics can help in the development of personalized treatment plans by predicting disease progression and response to treatment. The adoption of AI and ML in diagnostics is expected to revolutionize the market, making Hepatitis E detection more precise and accessible.
Hepatitis E Diagnostic Tests Market Segment Insights
Hepatitis E Diagnostic Tests Market Type Insights
The global Hepatitis E diagnostic tests market is segmented based on test types into ELISA HEV IgM Test, ELISA HEV IgG Test, Rapid Diagnostics Test, and RT-PCR. In 2023, the ELISA HEV IgM Test segment dominated the market. This dominance is attributed to its widespread use in detecting acute Hepatitis E infection, as it identifies the presence of IgM antibodies produced in response to the infection. The ELISA HEV IgM Test is favored for its reliability, cost-effectiveness, and ease of use in various clinical settings, particularly in developing regions where the prevalence of Hepatitis E is higher.
On the other hand, the RT-PCR segment is the highest-growing segment within the market. This growth is driven by the increasing demand for highly sensitive and specific diagnostic tools that can detect the presence of HEV RNA, enabling early and accurate diagnosis of the infection. RT-PCR is particularly valuable in identifying chronic HEV infections and in cases where antibody detection might not be sufficient. The rapid advancements in molecular diagnostic technologies and the growing adoption of RT-PCR in both developed and developing regions are contributing to its robust growth. Additionally, the trend towards more sophisticated diagnostics and the increasing focus on early disease detection is further propelling the demand for RT-PCR tests in the Hepatitis E diagnostic landscape.
Hepatitis E Diagnostic Tests Market End User Insights
The global Hepatitis E diagnostic tests market is segmented based on end-users into hospitals, diagnostic laboratories, research centers, and point-of-care. In 2023, hospital was the dominating segment in this market, primarily due to their extensive infrastructure, availability of advanced diagnostic equipment, and a high volume of patients requiring diagnostic services. Hospitals also often serve as the primary point of care for acute and severe cases of Hepatitis E, making them crucial in the initial diagnosis and management of the disease. Their comprehensive range of services and the ability to handle complex cases contribute significantly to their leading position in the market.
The point-of-care segment is the highest-growing segment within the market. This rapid growth is fueled by the increasing demand for convenient and quick diagnostic solutions, especially in remote and resource-limited settings. Point-of-care diagnostics offer the advantage of immediate results, which is crucial for timely decision-making and management of Hepatitis E cases. The growing emphasis on decentralizing healthcare, improving access to diagnostic services, and the development of user-friendly, portable testing devices are key factors driving the expansion of this segment. As healthcare systems worldwide continue to adopt more flexible and accessible diagnostic approaches, the point-of-care segment is expected to witness substantial growth.
Global Hepatitis E Diagnostic Tests Market, Segmental Coverage, 2019 - 2032 (USD Million)
Source: Secondary Research, Primary Research, PMR Database and Analyst Review
Hepatitis E Diagnostic Tests Market Regional Insights
By region, the study provides market insights into North America, Europe, Asia-Pacific, Latin America, and Middle East & Africa. North America and Europe are leading due to advanced healthcare infrastructure, high awareness, and significant investment in diagnostic technologies. Asia-Pacific is the fastest-growing region, driven by high disease prevalence, large populations, and improved healthcare systems in countries like China, India, and Japan. Latin America shows moderate growth with increasing awareness and healthcare improvements, particularly in Brazil and Mexico, despite economic constraints. The Middle East and Africa present a mixed outlook, with growth driven by countries like South Africa and Saudi Arabia, though disparities in healthcare access and infrastructure remain a challenge.
The major countries studied in the market report are the US, Canada, German, France, the UK, Italy, Spain, China, Japan, India, Australia, South Korea, and Brazil.
Europe’s Hepatitis E diagnostic tests market is growing due to increasing cases of the disease, particularly in countries like Germany, France, and the UK. The region's strong regulatory framework ensures high standards in diagnostic testing, promoting the use of reliable and advanced diagnostic methods. Public health campaigns and government support for disease surveillance and control are significant drivers. The market also benefits from collaborations between public health institutions and private companies to enhance diagnostic capabilities.
Asia-Pacific Hepatitis E diagnostic tests market is the fastest-growing region, driven by high disease prevalence, large populations, and improving healthcare infrastructure. China, India, and Japan’s Hepatitis E diagnostic tests market are key contributors, with increasing investments in healthcare and diagnostic technologies. Government initiatives to improve public health, coupled with rising awareness and accessibility of diagnostic services, are propelling market growth. The region's focus on enhancing healthcare infrastructure and expanding diagnostic capabilities in rural and urban areas is crucial.
Global Hepatitis E Diagnostic Tests Market Regional Coverage, 2019 - 2032 (USD Million)
Source: Secondary Research, Primary Research, PMR Database and Analyst Review
Hepatitis E Diagnostic Tests Market Key Market Players & Competitive Insights
Key players in the global Hepatitis E diagnostic tests market include Abbott Laboratories, F. Hoffmann-La Roche Ltd, Siemens Healthineers, Bio-Rad Laboratories, Thermo Fisher Scientific, DiaSorin S.p.A., Perkin Elmer Inc., QIAGEN N.V., bioMérieux SA, Merck KGaA, Ortho Clinical Diagnostics, InBios International, Altona Diagnostics, Luminex Corporation, and Randox Laboratories. These companies are at the forefront of developing innovative diagnostic solutions, offering a range of products from ELISA kits to advanced molecular diagnostics like RT-PCR.
The competitive landscape is marked by continuous innovation, strategic collaborations, and a focus on expanding geographic reach. Key players are investing in R&D to enhance the sensitivity and specificity of diagnostic tests while also seeking to make these tests more accessible in resource-limited settings. Partnerships with healthcare organizations and government bodies are common, aiming to improve disease surveillance and management. Overall, the market is highly dynamic, with ongoing efforts to address the global burden of Hepatitis E through improved diagnostic capabilities.
Abbott Laboratories is a leading player in the Hepatitis E diagnostic tests market, known for its comprehensive range of diagnostic products and solutions. The company’s diagnostic division offers advanced molecular diagnostics, immunoassays, and point-of-care testing kits that are widely used in detecting Hepatitis E virus (HEV) infections. Abbott's strong focus on innovation and R&D has resulted in the development of highly sensitive and specific diagnostic tests, contributing significantly to early disease detection and management.
F. Hoffmann-La Roche Ltd (Roche) is another major player in the Hepatitis E diagnostic tests market, renowned for its advanced diagnostic technologies and robust global presence. Roche offers a variety of diagnostic tools, including the COBAS systems for real-time PCR testing, which are highly effective in detecting HEV RNA. The company’s diagnostics solutions are recognized for their high precision and reliability, making them a preferred choice in both hospital and laboratory settings.
Key Companies in the Hepatitis E Diagnostic Tests Market include
- Abbott Laboratories
- F. Hoffmann-La Roche Ltd
- Siemens Healthineers
- Bio-Rad Laboratories
- Thermo Fisher Scientific
- DiaSorin S.p.A.
- Perkin Elmer Inc.
- QIAGEN N.V.
- bioMérieux SA
- Merck KGaA
- Ortho Clinical Diagnostics
- InBios International
- Altona Diagnostics
- Luminex Corporation
- Randox Laboratories
Hepatitis E Diagnostic Tests Industry Developments
- In November 2023, F. Hoffmann-La Roche Ltd launched automated serology hepatitis E virus tests named Elecsys Anti-HEV IgM and Elecsys Anti-HEV IgG immunoassays.
- In November 2023, WHO included HEV tests, such as the Altona assay, in their Essential Diagnostics List (EDL).
Hepatitis E Diagnostic Tests Market Segmentation
Hepatitis E Diagnostic Tests Type Outlook
- ELISA HEV IgM Test
- ELISA HEV IgG Test
- Rapid Diagnostics Test
- RT-PCR
Hepatitis E Diagnostic Tests End User Outlook
- Hospitals
- Diagnostic Laboratories
- Research Centers
- Point-of-Care
Hepatitis E Diagnostic Tests Regional Outlook
- North America
- US
- Canada
- Europe
- Germany
- France
- UK
- Italy
- Spain
- Netherlands
- Russia
- Rest of Europe
- Asia-Pacific
- China
- Japan
- India
- Malaysia
- South Korea
- Indonesia
- Australia
- Vietnam
- Rest of Asia-Pacific
- Middle East & Africa
- Saudi Arabia
- UAE
- Israel
- South Africa
- Rest of Middle East & Africa
- Latin America
- Mexico
- Brazil
- Argentina
- Rest of Latin America
-
Hepatitis E Diagnostic Tests Report Scope
|
Report Attributes |
Details |
|
Market size value in 2023 |
USD 53.05 million |
|
Market size value in 2024 |
USD 54.61 million |
|
Revenue Forecast in 2032 |
USD 78.62 million |
|
CAGR |
4.6% from 2024 – 2032 |
|
Base year |
2023 |
|
Historical Data |
2019 – 2022 |
|
Forecast Period |
2024 – 2032 |
|
Quantitative Units |
Revenue in USD million and CAGR from 2024 to 2032 |
|
Report Coverage |
Revenue Forecast, Market Competitive Landscape, Growth Factors, and Trends |
|
Segments covered |
|
|
Regional scope |
|
|
Competitive Landscape |
Hepatitis E Diagnostic Tests Market Share Analysis (2023) Company Profiles/Industry participants profiling includes company overview, financial information, product/service benchmarking, and recent developments |
|
Report Format |
|
|
Customization |
Report customization as per your requirements with respect to countries, regions, and segmentation. |
FAQ's
The global Hepatitis E diagnostic tests market size was valued at USD 53.05 million in 2023 and is projected to grow to USD 78.62 million by 2032.
The global market is projected to grow at a CAGR of 4.6% during the forecast period, 2024-2032.
North America had the largest share of the global market
The key players in the market are Abbott Laboratories, F. Hoffmann-La Roche Ltd, Siemens Healthineers, Bio-Rad Laboratories, Thermo Fisher Scientific, DiaSorin S.p.A., Perkin Elmer Inc., QIAGEN N.V., bioMérieux SA, Merck KGaA, Ortho Clinical Diagnostics, InBios International, Altona Diagnostics, Luminex Corporation, and Randox Laboratories.
The ELISA HEV IgM test category dominated the market in 2023.
The hospitals segment had the largest share in the global market.
