
Multi Cancer Early Detection Market Share, Size, Trends, Industry Analysis Report,
By Product (Bullets, Aerial Bombs, Grenades, Artillery Shells, Mortars); By Component; By Caliber; By Guidance Mechanism; By Application; By Lethality; By Region; Segment Forecast, 2024 - 2032
- Published Date:Jun-2024
- Pages: 116
- Format: PDF
- Report ID: PM4952
- Base Year: 2023
- Historical Data: 2019-2022
Report Outlook
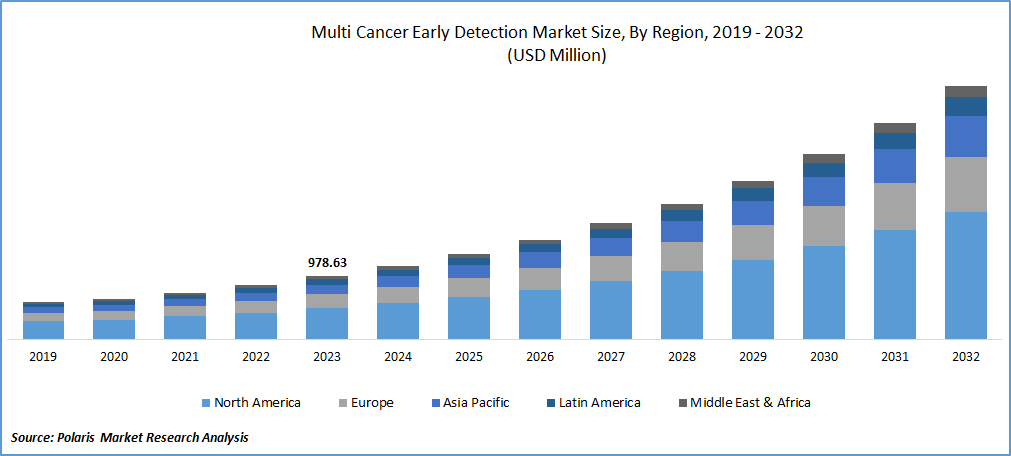
Multi Cancer Early Detection Market size was valued at USD 978.63 million in 2023. The market is anticipated to grow from USD 1,139.91 million in 2024 to USD 3,934.79 million by 2032, exhibiting the CAGR of 16.7% during the forecast period.
Market Overview
Current technological advancements have led to the development of multi-cancer early detection (MCED) screening tests, which use liquid biopsy technologies capable of identifying several types of cancer from blood samples. These screening tests aim to identify cancer-associated signals in their early stages, covering a spectrum of cancers, including those not typically targeted by standard screening modalities.
To Understand More About this Research:Request a Free Sample Report
Tests recently in development are expected to serve multiple purposes, including complementing existing standard screening procedures. Standard screening activities among individuals undergoing MCED screening tests are important to understanding the impact and implications of these recent technologies. They essentially influence shifts in screening activities and play a crucial role in the future of cancer prevention and detection technology.
- In February 2024, the NIH initiated a research network to evaluate emerging cancer screening technologies. This initiative supported the Biden-Harris administration's Cancer Moonshot agenda by exploring methods to detect cancers earlier, potentially making them more manageable to treat.
Blood-based multi-cancer early detection tests hold the potential to improve cancer survival rates by expanding population screening efforts. Leveraging genome sequencing and machine learning, these tests have been developed to complement single-cancer screening techniques. By analyzing circulating cell-free DNA, this multi-cancer early detection (MCED) test can detect common cancer signals among several types of tumors.
- For instance, in September 2023, the Eone-Diagnomics Genome Center (EDGC) is poised to introduce a blood-based multi-cancer early detection test. The aim was to scale up this test to detect numerous cancer types and explore potential applications beyond cancer detection.
Growth Drivers
Multi-Cancer Early Detection (MCED) Tests Transforming Cancer Screening with AI Integration
In the healthcare sector, MCED tests have become an important screening tool, with molecular analysis of tumor-related indicators in bodily fluids with advanced artificial intelligence (AI) algorithms. This novel technique enables the simultaneous detection of various cancers while precisely determining the specific cancer type. Rapidly advancing AI technology, the merging of MCED with AI has emerged as a prominent trend, encouraging the development of a diverse range of MCED AI products. This convergence creates new opportunities for personalized medicine and patient care while also improving the effectiveness of cancer screening.
Enhancing Cancer Detection Rates
MCED tests are designed with the purpose of maintaining high specificity levels while efficiently detecting common signals present in several types of cancer. This accuracy makes a notable increase in the total cancer detection rate within the population. By implementing multi-cancer early detection strategies, businesses stand poised to significantly increase cancer detection rates within general populations, preferably identifying cases at earlier stages when treatment options and prognoses are more favorable.
- Additionally, in November 2023, GRAIL initiated the REACH Study to evaluate the clinical impact of the Galleri Multi-Cancer Early Detection (MCED) Test among the Medicare population.
Restraining Factors
Potential Risks and Uncertainties Surrounding Multi-Cancer Early Detection Tests
Despite the promising potential of multi-cancer early detection tests, several risks and uncertainties restrain their widespread adoption. The high cost of development and testing can limit accessibility and affordability. Moreover, regulatory hurdles and the need for extensive clinical validation pose significant challenges. False positives and negatives also present risks, potentially leading to unnecessary treatments or missed diagnoses. Additionally, ethical concerns regarding patient privacy and data security complicate implementation. These factors, coupled with varying healthcare infrastructure and reimbursement policies, create uncertainties that must be addressed to realize the benefits of multi-cancer early detection technologies fully.

Report Segmentation
The market is primarily segmented based on type, end user and region.
|
By Type |
By End User |
By Region |
|
|
|
To Understand the Scope of this Report:Speak to Analyst
By Product Analysis
The gene panel, LDT segment held the largest share of the market during the forecast period. This dominance is due to the incorporation of clinically target sequences into NGS-based gene panel assays, which resulted in practical diagnostic tools that facilitate personalized cancer patient care. It offers clinical utility by examining multiple aspects, including the genetic basis for an individual's response to therapy, like signaling pathways linked to specific treatments, microsatellite instability, hypermutated phenotypes, and deficiencies in the DNA double-strand break repair pathway.
The liquid biopsy segment is poised for rapid growth over the forecast period. This growth is due to the pivotal role of liquid biopsies in early detection in improving quality of life, survival rates, and reducing the cost burden of cancer therapies. There is a pressing need for liquid biopsies with heightened sensitivity.
By End Use
In 2023, the hospitals segment held the largest share. This is because MCEDs in hospitals play an important role in providing comprehensive diagnostic assessments in patients with positive MCED test results. MCED innovative tests screen for a multitude of cancers, including colorectal, breast, cervical, and prostate cancers, with a single blood sample.
Diagnostic laboratories will grow rapidly due to the main use of blood samples, offering several advantages for screening as an emerging health technology. Multi-cancer early detection technologies have the potential to interrupt conventional models of cancer screening, offering significant opportunities for diagnostic laboratories to adapt and capitalize on this evolving landscape.
Regional Insights`
North America dominated the global market in 2023. The North America multi-cancer early detection market is experiencing significant growth, driven by advancements in diagnostic technologies and a rising emphasis on early cancer detection. This market encompasses a range of innovative diagnostic tests designed to detect multiple types of cancer at early stages using various biomarkers, including genetic, proteomic, and epigenetic markers. The objective is to improve patient outcomes through early intervention and more effective treatment strategies.
Several driving factors are propelling the growth of this market. Firstly, the increasing prevalence of cancer in North America underscores the urgent need for early detection methods. According to the American Cancer Society, there are an estimated 1.8 million new cancer cases diagnosed annually in the United States alone, highlighting the critical demand for advanced diagnostic tools. Secondly, technological advancements in genomic and molecular diagnostics have significantly enhanced the accuracy and reliability of multi-cancer detection tests. These innovations enable the identification of cancer at much earlier stages than traditional methods, often before symptoms appear.
Furthermore, substantial investments in research and development by both public and private sectors are accelerating the commercialization of multi-cancer early detection tests. Government initiatives and funding aimed at improving cancer screening programs contribute to market expansion. Additionally, growing awareness among healthcare providers and patients about the benefits of early cancer detection is fostering the adoption of these advanced diagnostic tools.
Trends Key Market Players & Competitive Insights
Strategic partnerships to drive the competition
The multi cancer early detection market is fragmented. The ongoing expansion initiatives, including partnerships, acquisitions, and collaborations, are fueling competition in the marketplace. For instance, in July 2023, PredOmix Technologies & CORE Diagnostics collaborated to launch OncoVeryx-F, a state-of-the-art multi-cancer early detection test designed for high-risk & asymptomatic women.
Some of the major players operating in the global market include:
- Illumina, Inc. (GRAIL, LLC)
- Exact Sciences Corp.
- Foundation Medicine, Inc.
- AnchorDx
- Guardant Health
- Burning Rock Biotech Ltd.
- GENECAST
- Beijing Lyman Juntai International Medical Technology Development Co.
- Freenome Holdings, Inc.
- Elypta AB
Recent Developments in the Industry
- In April 2023, Illumina was ordered by the FTC to divest GRAIL, a cancer detection test maker, in order to safeguard competition within the life-saving technology market.
- In April 2024, RenovaroCube, a subsidiary of Renovaro, unveiled Flamingo, a novel AI model for multi-cancer detection utilizing ultra-low pass whole genome sequencing of cell-free DNA.
Report Coverage
The multi cancer early detection market report emphasizes on key regions across the globe to provide better understanding of the product to the users. Also, the report provides market insights into recent developments, trends and analyzes the technologies that are gaining traction around the globe. Furthermore, the report covers in-depth qualitative analysis pertaining to various paradigm shifts associated with the transformation of these solutions.
The report provides detailed analysis of the market while focusing on various key aspects such as competitive analysis, product, component, caliber, guidance mechanism, application, lethality, and their futuristic growth opportunities.
Multi Cancer Early Detection Market Report Scope
|
Report Attributes |
Details |
|
Market size value in 2024 |
USD 1,139.91 million |
|
Revenue forecast in 2032 |
USD 3,934.79 million |
|
CAGR |
16.7% from 2024 – 2032 |
|
Base year |
2023 |
|
Historical data |
2019 – 2022 |
|
Forecast period |
2024 – 2032 |
|
Quantitative units |
Revenue in USD million and CAGR from 2024 to 2032 |
|
Segments covered |
|
|
Regional scope |
|
|
Competitive Landscape |
|
|
Report Format |
|
|
Customization |
Report customization as per your requirements with respect to countries, region and segmentation. |
FAQ's
Multi Cancer Early Detection Market Size Worth $ 3,934.79 Million By 2032
The top market players in Multi Cancer Early Detection Market are Illumina, Sciences Corp., Foundation Medicine
North America contribute notably towards the Multi Cancer Early Detection Market
Multi Cancer Early Detection Market exhibiting the CAGR of 16.7% during the forecast period
Multi Cancer Early Detection Market report covering key segments are type, end user and region
